Molecular mechanism of membrane-associated folding and unfolding
We study thermodynamics and kinetics of polypeptide interaction with lipid bilayer of membrane to elucidate the molecular mechanism of membrane-associated folding and unfolding. Our model system is pHLIP®s (pH Low Insertion Peptides), which can undergo transitions triggered by changes of pH. We were able to follow changes in CD and fluorescence during pHLIP®s insertion into membrane and folding, as well as exit and unfolding within msec/sec time scale. Partition of the peptide into bilayer triggers formation of helical structure, while exit promotes unfolding. The existence of membrane- surface helical intermediate along the folding pathway is not mandatory, however, it could be stabilized if polypeptide inserting end would carry charges or polar groups. Our goal is to introduce physical model of membrane-associated folding/unfolding based on the experimental results and guided by the approaches of statistical physics, kinetic theory and molecular dynamic calculations in collaboration with our partners.
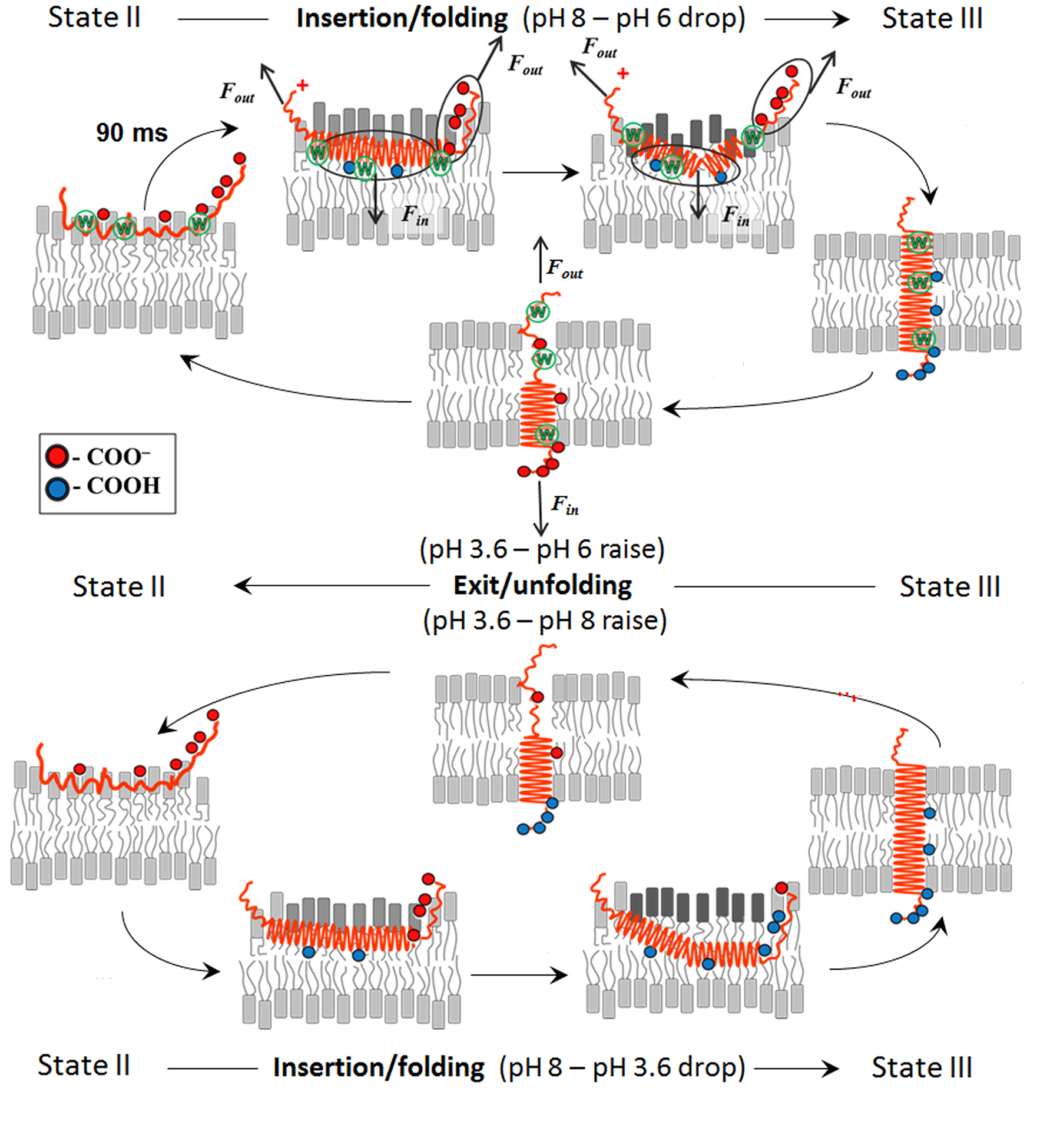 |
Schematic presentation of molecular mechanism of membrane-associated polypeptide folding and unfolding triggered by pH changes. |
