pHLIP Technology
The results of biophysical studies led to the rational design of a novel class of delivery agents (pHLIPs®), which can selectively target and deliver diagnostic and therapeutic molecules to acidic diseased tissues.
pHLIP® for imaging and fluorescence-guided surgical interventions pHLIP® peptides labeled with optical, PET or SPECT probes are considered to be the first acidity markers. We have shown targeting of diseased acidic tissue including primary tumors of various origins and metastatic lesions, ischemia, inflammatory arthritis on animal models and targeting of cancerous lesions on human tissue specimens. We proved that targeting is indeed pH-dependent. Metastatic and aggressive tumors, which are more acidic, are targeted more efficiently compared to non-metastatic ones, and tumor margins are stained with high accuracy. Currently, pHLIP® imaging agents are in the process of clinical translation for PET diagnostic imaging of tumor acidity and fluorescence-guided surgery. pHLIP® for drug delivery The membrane-associated folding of pHLIP® is accompanied by a release of free energy. We demonstrated that this energy can be used to move polar, cell-impermeable cargo molecules across the membrane into a cell. Such translocation is selective for low pH, and various types of cargo molecules attached by cleavable to the inserting end of pHLIP® have thus been transported into cells and released in the cytoplasm. Among translocated cargo molecules are fluorescent dyes, toxins, peptides and gene- regulation agents. The polar and moderately hydrophobic molecules are moved across membrane, find their cellular target and induce desired biological effect. The approach opens novel direction in drug delivery.
 |
Images of tumor spheroids and Trypan Blue assay (A). Fluorescence images of HeLa tumor spheroids treated with the SNARF pHLIP ® peptide at pH 6.6 were acquired using 580 nm and 640 nm emission filters. The SNARF pHLIP ® peptide images of HeLa tumor spheroids are shown before and immediately after the addition of cell-impermeable Trypan Blue, which clearly demonstrates extracellular localization of SNARF fluorophore when pHLIP ® is inserted into plasma membrane of cells. pH measured at the surfaces of cancer cells in tumors in vivo (B). Fluorescence spectra recorded from tumors in live mice (skin is removed from the tumor site) 4 hours after administration of SNARF pHLIP ® peptide as a single tail vein injection before and after IP injection of 125 mg of glucose. pH imaging of cancer cells ex vivo (C). Fluorescence image obtained from a HeLa tumor specimen treated ex vivo with the SNARF pHLIP ® peptide in the presence of glucose, followed by washing. Images are from paper: Anderson et al, 2016, Proc Natl Acad Sci U S A. |
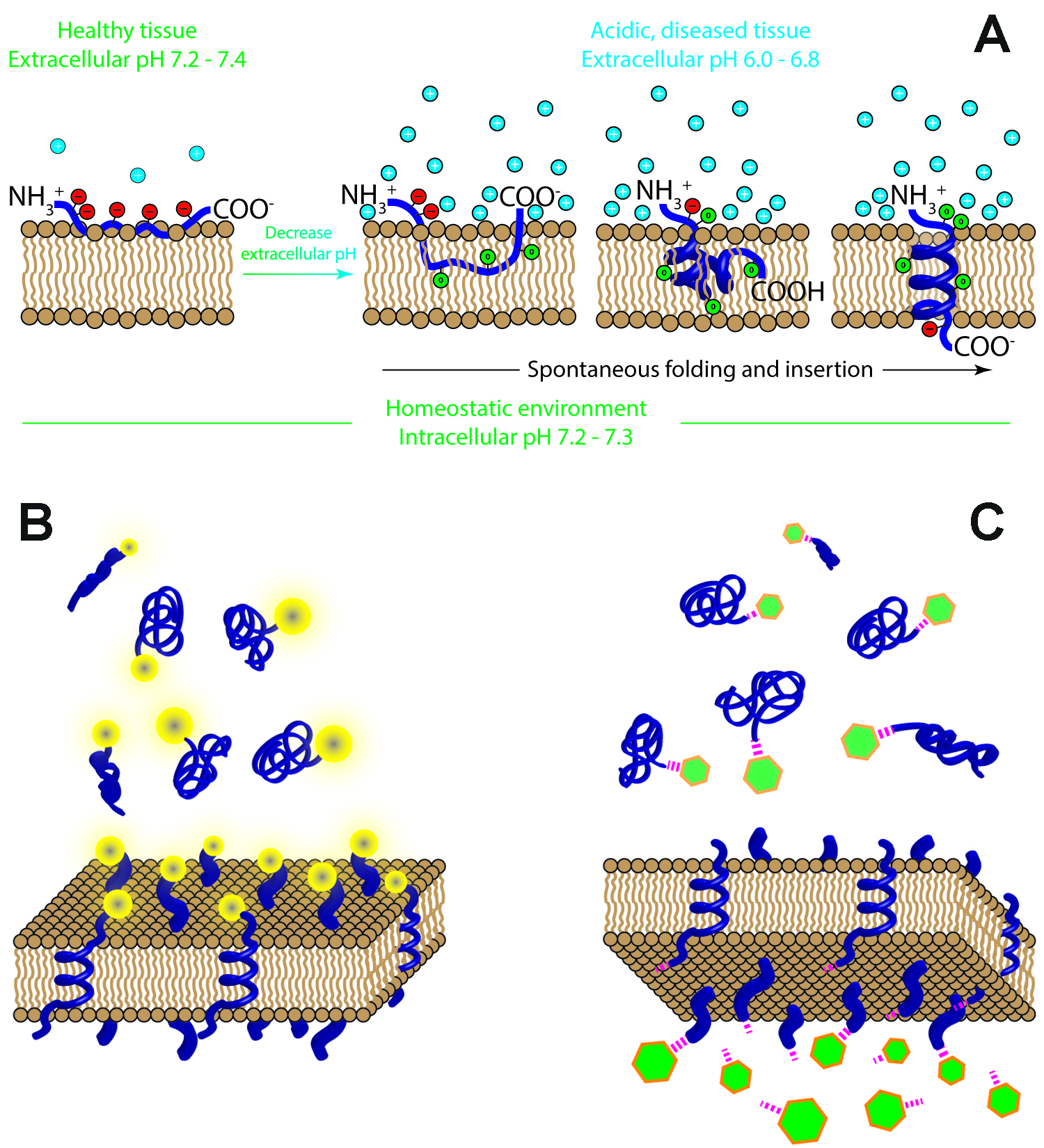
 |
Mechanism of pHLIP insertion into the cellular membrane (A). When the pHLIP (blue) encounters healthy tissue where the extracellular pH is around pH 7.4, the protonatable residues of the pHLIP (red circles) remain deprotonated and negatively charged, and the peptide resides at or near the hydrophilic surface of the cellular membrane. Weakly bound to the membrane, the pHLIP is washed from the membrane via normal perfusion and continues to circulate through the body. Cancer cells, however, produce excess acidity as a consequence of their malfunctioning metabolisms and overexpression of certain surface proteins, and pump these acidic byproducts out of the cell interior in order to maintain comfortable conditions inside the cell, resulting in the acidification of tumor tissue. When the pHLIP encounters tumor tissue, it senses the low extracellular pH at the cancer cell surface (i.e., the concentration of protons (cyan circles) at the surface of the cellular membrane is high), and the protonatable residues and negatively charged C-terminal carboxyl group of the pHLIP become neutrally charged (green circles). The protonation leads to an increase in the overall hydrophobicity of the pHLIP, increasing the affinity of the peptide to the hydrophobic core of the cellular membrane and triggering the pHLIP to spontaneously fold into a helix and insert across the membrane, resulting in the formation of a transmembrane helix. When the C-terminal protonatable residue and carboxyl group are then exposed to the normal intracellular pH of the cell, they are deprotonated, again becoming negatively charged, and anchor the pHLIP in the membrane. Tethering cargo to the cell surface (B). A pHLIP can be used to target and tether cargo molecules to the surfaces of cells in low pH environments. The cargo could be an optical marker, a PET or SPECT imaging agent, or an antigen or protein delivered to induce certain cellular processes. Translocating cargo across the membrane into the cytoplasm (C). A pHLIP can also be used for the intracellular delivery of payloads, translocating cargo (green) across the membranes of cells with low extracellular pH, such as those cells found in acidic, diseased tissue. These payloads are conjugated to the membrane-inserting end of the pHLIP, typically via a cleavable link (magenta), and could include toxins, chemotherapeutic agents, or agents to alter gene expression. The figures are from Review paper: Wyatt et al., 2018, Trends Biotechnology. |
